

Up: The chemistry of the
Subsections
142 lines originating from a total of 19 species were detected and are shown in Table
5. 6 further lines could not be identified and are indicated as such by U in Table
5. Many isotopomers were detected, particularly the 34SO2 isotopomer. A list of the
identified species (including isotopomers) and the total number of lines from each is given in Table
1. Approximately
50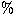 of the identified lines originate from sulphur-bearing species. Many of these lines (e.g. CS, SO,
SO2 and to some extent C34S and 34SO) exhibit broad line wings suggesting that their
emission originates from regions of wide velocity dispersion, perhaps from the molecular outflow. Other
non sulphur-bearing species known as typical outflow tracers (CO, HCO+, CH3OH) also display a wide
range of velocities. Isotopomers such as 13CO, H13CO+ and C34S have broad line
wings, which
indicates that the outflowing gas has a significant optical depth in these molecules.
The density tracer HCN also appears to have broad line wings, although this is complicated by the
presence of two
image band SO2 lines blended with the HCN 4-3 line. SiO exhibits extremely broad emission (across
of the identified lines originate from sulphur-bearing species. Many of these lines (e.g. CS, SO,
SO2 and to some extent C34S and 34SO) exhibit broad line wings suggesting that their
emission originates from regions of wide velocity dispersion, perhaps from the molecular outflow. Other
non sulphur-bearing species known as typical outflow tracers (CO, HCO+, CH3OH) also display a wide
range of velocities. Isotopomers such as 13CO, H13CO+ and C34S have broad line
wings, which
indicates that the outflowing gas has a significant optical depth in these molecules.
The density tracer HCN also appears to have broad line wings, although this is complicated by the
presence of two
image band SO2 lines blended with the HCN 4-3 line. SiO exhibits extremely broad emission (across
 ), however it is again unfortunately blended with a vibrational SO2
line in the image band.
), however it is again unfortunately blended with a vibrational SO2
line in the image band.
Table 1:
Species (including isotopomers) identified in the survey.
The identification of certain species from single
line detections must however be regarded with caution
| |
In contrast to the broad lines thought to have an origin within the outflow, several species with narrow
line profiles (e.g. HC3N, CH3CN, CH3CCH, NO) trace a
relatively quiescent region of gas, probably the molecular envelope surrounding the UC HII region
(Gomez
et al. 1991). Figure 2 shows a selection of high-velocity lines compared
with the CH3CCH 20(2)-19(2) line as representative of the low velocity dispersion lines. The
temperature scale is normalised to highlight the differences between them.
The differences between the narrow CH3CCH line which probes the gas of the molecular envelope and
the broad lines of the outflow tracers SO, SO2 and HCO+ are obvious. The CH3OH line
also has a distinct red wing.
![\begin{figure}
\includegraphics [width=8cm]{ds1633f2.eps}\end{figure}](/articles/aas/full/1999/06/ds1633/Timg26.gif) |
Figure 2:
Spectra of broad lines
from species known to probe outflows are compared to the
CH3CCH 20(2)-19(2) line. Broad red and
blue-shifted wings are clearly visible in the SO, SO2 and HCO+ lines,
whereas the CH3OH 7(0)-6(0) A+
line only clearly exhibits a red-shifted wing |
In contrast to other high-mass YSO sources such as G34.26+0.15 and Orion-KL, there is no observational
evidence of the presence of heavy organic molecules such as ethyl cyanide (CH3CH2CN),
methyl formate (HCOOCH3) or dimethyl ether (CH3OCH3). These molecules have large numbers
of emission lines detected towards the other sources and are evidence of a complex hot core chemistry,
which may be absent in the molecular gas associated with G5.89-0.39.
We have also compared the total integrated intensities from each molecule, in a similar manner to that of
the Orion-KL survey (Schilke et al. 1997b). It is interesting to compare the results from G5.89 with
those of Orion-KL. In Orion-KL the most dominant molecule in terms of integrated intensity is SO2
which has a total integrated intensity more than twice that of CO. In G5.89-0.39 the most dominant
molecule is CO with twice the integrated intensity of SO2. This is due to beam dilution,
since G5.89-0.39 is approximately 4 times more distant than Orion KL. The same effect can be seen in other
species such as CH3CCH and CH3CN; in Orion-KL the former is much less important for cooling the
gas than the latter. However in G5.89 CH3CCH has twice the integrated intensity of CH3CN. This
is probably due to the property of CH3CCH of tracing more extended gas (by virtue of its lower dipole
moment), making CH3CCH less affected by beam dilution. Care must be taken in interpretations such as
this though, as genuine chemical differences may affect the relative abundances of particular species between the
two clouds and
hence their integrated intensities.
Table 2:
Integrated intensity for each species. The integrated intensities have been evaluated using the
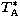 scale. Where it has not been possible to place a firm value on the total integrated intensity due
to blended lines an upper limit is indicated
scale. Where it has not been possible to place a firm value on the total integrated intensity due
to blended lines an upper limit is indicated
|
|
The rotation diagram approach assumes optically thin gas in LTE, for which the (beam-averaged)
column density (N) can be written as:
| 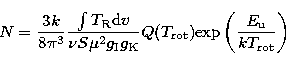 |
(1) |
where  is the integrated intensity of the line,
is the integrated intensity of the line,  is the line frequency, S is the line
strength,
is the line frequency, S is the line
strength,  is the
permanent electric dipole moment,
is the
permanent electric dipole moment,
 and
and  are the reduced nuclear spin degeneracy
and the K-level degeneracy of the molecule respectively.
are the reduced nuclear spin degeneracy
and the K-level degeneracy of the molecule respectively.  is the energy of the upper level of the line and
is the energy of the upper level of the line and
 is the rotational temperature of the molecules comprising the gas and
is the rotational temperature of the molecules comprising the gas and  is the corresponding partition function. Values for
is the corresponding partition function. Values for  were obtained by
interpolating the values given
in the JPL molecular line database to the appropriate temperature.
Equation (1) was rearranged to an equation for a straight line and
were obtained by
interpolating the values given
in the JPL molecular line database to the appropriate temperature.
Equation (1) was rearranged to an equation for a straight line and
 and N for each species were determined by a least-squares fit. A rotation diagram for
33SO was not constructed owing to the lack of well-determined molecular parameters, such as the
partition function.
Lower limits to the column densities (
and N for each species were determined by a least-squares fit. A rotation diagram for
33SO was not constructed owing to the lack of well-determined molecular parameters, such as the
partition function.
Lower limits to the column densities ( ) of the remaining species were evaluated
using the minimum point of Eq. (1), which occurs at
) of the remaining species were evaluated
using the minimum point of Eq. (1), which occurs at  for linear molecules and
for linear molecules and  for symmetric and asymmetric top
molecules.
Both methods are described more fully in Hatchell et al. (1998a).
for symmetric and asymmetric top
molecules.
Both methods are described more fully in Hatchell et al. (1998a).
The results of the rotation diagram and lower
limit analyses are given in Tables
3 and 4. Abundances have been calculated for the rotation diagram species by
assuming a spherical cloud with a radius of 0.2 pc and a number density  = 104 cm-3 (taken
from Gomez et al. 1991). The abundance of 34SO2 indicates that the SO2 abundance may be underestimated
due to optical depth effects and should be closer to 3 106. The rotation diagrams are shown in
Fig. 3. Certain lines have been excluded from the analysis; self-absorbed and blended
lines, and those lines that could not be unambiguously identified with a single species. The
data from SO, 34SO and H2CS did not give a satisfactory fit
to a straight line and lower limits to column density have been evaluated for these molecules.
= 104 cm-3 (taken
from Gomez et al. 1991). The abundance of 34SO2 indicates that the SO2 abundance may be underestimated
due to optical depth effects and should be closer to 3 106. The rotation diagrams are shown in
Fig. 3. Certain lines have been excluded from the analysis; self-absorbed and blended
lines, and those lines that could not be unambiguously identified with a single species. The
data from SO, 34SO and H2CS did not give a satisfactory fit
to a straight line and lower limits to column density have been evaluated for these molecules.
![\begin{figure}
\includegraphics [height=22cm,scale=0.5]{ds1633f3.eps}\end{figure}](/articles/aas/full/1999/06/ds1633/Timg41.gif) |
Figure 3:
Rotation diagrams for species with sufficient detected lines |
The rotation diagrams indicate that the molecular gas associated with G5.89 has a temperature of
roughly 60-70 K and a column density of
 cm-2. SO2 appears to probe
somewhat hotter gas with temperature in excess of 100 K. The temperature for the less optically
thick 34SO2 is higher than that of SO2 which is consistent with (although not
conclusive evidence for) the temperature
of the molecular gas increasing towards the centre.
cm-2. SO2 appears to probe
somewhat hotter gas with temperature in excess of 100 K. The temperature for the less optically
thick 34SO2 is higher than that of SO2 which is consistent with (although not
conclusive evidence for) the temperature
of the molecular gas increasing towards the centre.
Table 3:
Rotation temperatures ( ), partition functions (
), partition functions ( ),
column densities (N) and abundances (X) relative to H2.
Abundances were calculated assuming a spherical envelope with the radius and number density
),
column densities (N) and abundances (X) relative to H2.
Abundances were calculated assuming a spherical envelope with the radius and number density  given in
Gomez et al. (1991)
given in
Gomez et al. (1991)
|
|
Table 4:
Lower limits to column density. The partition function Q evaluated at
 is also given, where
is also given, where
 for linear molecules
and
for linear molecules
and  for symmetric and asymmetric tops. Where two lines
were used to evaluate the lower limit the
partition functions of both lines are given
for symmetric and asymmetric tops. Where two lines
were used to evaluate the lower limit the
partition functions of both lines are given
|
|
Table 5:
The measured line parameters of observed frequency ( (obs)), peak temperature (
(obs)), peak temperature (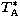 ) and line
width (
) and line
width ( ) for each detected line are listed here. Multiple detections of the same line have been
included. Lines that are blended are indicated in the Notes column by blended if they are blended with a different
species or h/fines if they are a mixture of two or more hyperfine components. Self-absorbed lines are also
listed. Blended lines that can have one or both components extracted are
indicated by sl-blend
) for each detected line are listed here. Multiple detections of the same line have been
included. Lines that are blended are indicated in the Notes column by blended if they are blended with a different
species or h/fines if they are a mixture of two or more hyperfine components. Self-absorbed lines are also
listed. Blended lines that can have one or both components extracted are
indicated by sl-blend

|
Table 5:
continued

|
Table 5:
continued

|
Table 5:
continued

|
Table 5:
continued

|
Table 5:
continued

|


Up: The chemistry of the
Copyright The European Southern Observatory (ESO)

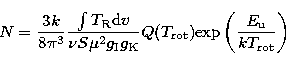
![]() = 104 cm-3 (taken
from Gomez et al. 1991). The abundance of 34SO2 indicates that the SO2 abundance may be underestimated
due to optical depth effects and should be closer to 3 106. The rotation diagrams are shown in
Fig. 3. Certain lines have been excluded from the analysis; self-absorbed and blended
lines, and those lines that could not be unambiguously identified with a single species. The
data from SO, 34SO and H2CS did not give a satisfactory fit
to a straight line and lower limits to column density have been evaluated for these molecules.
= 104 cm-3 (taken
from Gomez et al. 1991). The abundance of 34SO2 indicates that the SO2 abundance may be underestimated
due to optical depth effects and should be closer to 3 106. The rotation diagrams are shown in
Fig. 3. Certain lines have been excluded from the analysis; self-absorbed and blended
lines, and those lines that could not be unambiguously identified with a single species. The
data from SO, 34SO and H2CS did not give a satisfactory fit
to a straight line and lower limits to column density have been evaluated for these molecules.
![]() cm-2. SO2 appears to probe
somewhat hotter gas with temperature in excess of 100 K. The temperature for the less optically
thick 34SO2 is higher than that of SO2 which is consistent with (although not
conclusive evidence for) the temperature
of the molecular gas increasing towards the centre.
cm-2. SO2 appears to probe
somewhat hotter gas with temperature in excess of 100 K. The temperature for the less optically
thick 34SO2 is higher than that of SO2 which is consistent with (although not
conclusive evidence for) the temperature
of the molecular gas increasing towards the centre.
