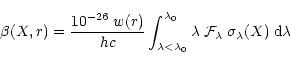Up: The UMIST database for 1999
Subsections
4 Other data
A comprehensive description of deuterium chemistry requires that all D-bearing
analogues of H-bearing species are included in a chemical model. This has the
effect of approximately tripling the number of reactions in a model if it is
to include deuterium. For our purposes, we have
decided not to include a full deuterium chemistry in this release. Such a
chemistry can be generated most efficiently using software but the detailed
branching ratios adopted are a matter of individual choice at this time
and we prefer to list only the most important reactions in
Table 6. Rodgers & Millar (1996) have discussed many of the
issues involved in generating a deuterium chemistry.
Table 5:
List of the types of chemical reactions and their position in
the database. The unclassifiable reactions are put in the category
"Sundries''. The first column XX is the abbreviation used for the types
|
XX |
Type of |
Position |
|
| |
reaction |
(index "I'') |
|
|
NN |
Neutral - Neutral Reactions |
1 |
- | 433 |
| IN |
Ion - Neutral Reactions |
434 |
- | 2606 |
| CE |
Charge Exchange Reactions |
2607 |
- | 3144 |
| II |
Ion - Ion Neutralisations |
3145 |
- | 3175 |
| DR |
Dissociative Recombinations |
3176 |
- | 3606 |
| RR |
Radiative Recombinations |
3607 |
- | 3631 |
| AD |
Associative Detachments |
3632 |
- | 3678 |
| RA |
Radiative Associations |
3679 |
- | 3760 |
| PH |
Photoprocesses |
3761 |
- | 3916 |
| CP |
Cosmic-Ray Proton Reacs (CRP) |
3917 |
- | 3927 |
| CR |
Cosmic-Ray Photon Reacs (CRPHOT) |
3928 |
- | 4059 |
| CL |
Collider Reactions |
4060 |
- | 4077 |
| TR |
Termolecular Reactions |
4078 |
- | 4107 |
| - |
Sundries |
4108 |
- | 4113 |
Table 7:
List of the species for which photo processes can be derived using known cross
sections. The photo processes are calculated only from the ground state
| Species |
Processes |
Products |
References |
| He |
ionisation |
He+ + e- |
Band et al. 1990 |
| C |
ionisation |
C+ + e- |
Cantù et al. 1981,
Hofmann et al. 1983 |
| C+ |
ionisation |
C++ + e- |
Henry 1970 |
| C++ |
ionisation |
C3+ + e- |
Osterbrock 1974 |
| N |
ionisation |
N+ + e- |
Henry 1970 |
| O |
ionisation |
O+ + e- |
Taylor & Burke 1976,
Henry 1970 |
| Ne |
ionisation |
Ne+ + e- |
Henry 1970 |
| Si |
ionisation |
Si+ + e- |
Chapman & Henry 1972 |
| C2 |
ionisation |
C2+ + e- |
Padial et al. 1985 |
| |
dissociation |
C + C |
Pouilly et al. 1983 |
| CO |
ionisation |
CO+ + e- |
Hudson 1971 |
| |
dissociation |
C + O |
Letzelter et al. 1987 |
| CO2 |
ionisation |
CO2+ + e- |
Hudson 1971, Hitchcock
et al. 1980 |
| |
fragmentation |
CO + O+ + e- |
Hitchcock et al. 1980 |
| |
|
O + CO+ + e- |
Hitchcock et al. 1980 |
| |
|
O2 + C+ + e- |
Hitchcock et al. 1980 |
| O2 |
ionisation |
O2+ + e- |
Brion & Tan 1979,
Ogawa & Ogawa 1975, |
| |
|
|
Clarke & Wayne 1970 |
| |
fragmentation |
O + O+ + e- |
Brion & Tan 1979 |
| N2O |
ionisation |
N2O+ + e- |
Hitchcock et al. 1980 |
| |
fragmentation |
NO+ + N + e- |
Hitchcock et al. 1980 |
| |
fragmentation |
NO + N+ + e- |
Hitchcock et al. 1980 |
| |
fragmentation |
N2+ + O + e- |
Hitchcock et al. 1980 |
| |
fragmentation |
N2 + O+ + e- |
Hitchcock et al. 1980 |
The necessity for having data on photo process cross sections arises from
the fact that astrochemistry is not exclusive to the ISM but is now
applied to circumstellar regions and comets as well. As a result, the rates
of destruction of the species through exposure to the stellar radiation
field can change by several orders of magnitude. For example, the
photoionization rate of
He0 in interstellar clouds is negligible, whereas in the radiation field of
a nearby Wolf-Rayet star it can be as large as 10-5 s-1.
Table 7 gives the list of the species and the data
references compiled to date by us. The detailed cross-sections, which are
available electronically, are given in megabarns (1 Mb = 10-18 cm2).
The photorate,
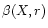 (s-1), for species X at a distance
r from a localised radiation source can be derived using
Eq. (5):
(s-1), for species X at a distance
r from a localised radiation source can be derived using
Eq. (5):
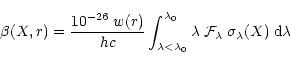 |
(5) |
where w(r) is the dilution factor at the distance r from the source,
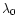 (Å) is the photoprocess threshold wavelength, such that
any photon with
(Å) is the photoprocess threshold wavelength, such that
any photon with
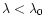 will take part to the process,
will take part to the process,
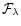 is the source Spectral Energy Distribution in erg cm-2 s-1 Å
-1,
is the source Spectral Energy Distribution in erg cm-2 s-1 Å
-1,
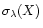 is the cross section (Mb) for the species
X at the wavelength
is the cross section (Mb) for the species
X at the wavelength 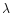 (Å).
(Å).
These data are not complete. Future work is aimed at updating these via the Opacity Project and other sources.
At low temperatures, the rates of ion-molecule reaction rate coefficients
can increase
dramatically through ion-dipole interactions, which are particularly important
for the interaction of light ions with neutrals possessing a permanent
electric dipole moment in excess of about 1 Debye. Table 3 gives electric dipole
moments for the neutral molecules contained in this database.


Up: The UMIST database for 1999
Copyright The European Southern Observatory (ESO)