

Up: The UMIST database for 1999
Subsections
3 The reaction set
Table 8 contains all the reactions and associated rate coefficients, and is available
online at
http://www.rate99.co.uk
http://www.edpsciences.org
3.1 The new entry format
The number of observed and predicted astrophysical species increases
steadily with
time, and with it, the size of their formula (e.g. ethyl methyl ether, C2H5OCH3, has been discussed as a possible interstellar molecule).
To account for this, we have altered the format for the names of the species
by increasing by one
letter, making them 8-character strings. Also, the smallest products in
a four-product reaction are mostly H and/or He, therefore only 4 characters
have been allocated for the last two products.
The necessity to consider termolecular reactions for high density
environments means that a third species must be included on the reactant
side of these reactions. To account for that, each reaction now comprises
three reactants and four products.
Our new reaction format reads:
I, R1, R2, R3, P1, P2, P3, P4, 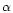 ,
,
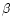 ,
,
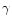 ,
flags
,
flags
where I is the reaction number, R1 to R3 are the reactants, P1 to P4 are
the products, and 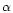 ,
,
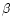 and
and 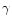 are the constants used to
determine the rate coefficients. The series of flags is a string of 16 characters and/or digits that store
respectively:
are the constants used to
determine the rate coefficients. The series of flags is a string of 16 characters and/or digits that store
respectively:
- the kind of data: measured M, estimated E, calculated C or
literature search L, with format A1. Here "literature search'' means that
the given datum
is a compilation of several other data (measured and/or calculated). The
sources of these data are mainly Baulch et al. (1992) and the NIST
database (Mallard et al. 1998);
- the lowest and highest temperatures defining the temperature range,
format 2(I5). Each temperature is given as an integer number of kelvins
in the range
10 < T < 41 000 K;
- the error on the rate value, format A1. The following scheme has been
used:
- "A''. Error < 25%
- "B''. Error < 50%
- "C''. Error within a factor of 2
- "D''. Error within an order of magnitude
- "E''. Highly uncertain;
- the reference code, format A4. The references are listed in
Table 4.
The full entry format in Fortran is correspondingly written as:
I4, 5(1X, A8), 2(1X, A4), 1X, 1PE8.2, 3X, 0PF5.2, 2X, 0PF8.1, A1, 2(I5), A1,
A4.
3.2 Calculation of the rates from 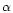 ,
,
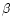 and
and 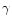
For two- or three-body reactions, the rate coefficient is given by:
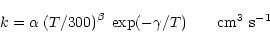 |
(1) |
where T is the gas temperature.
For direct cosmic-ray ionisation (R2 = CRP):
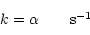 |
(2) |
whereas for cosmic-ray-induced photoreactions (R2 = CRPHOT):
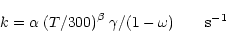 |
(3) |
where 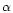 is the cosmic-ray ionisation rate,
is the cosmic-ray ionisation rate, 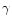 is the
probability per cosmic-ray ionisation that the appropriate photoreaction takes
place, and
is the
probability per cosmic-ray ionisation that the appropriate photoreaction takes
place, and 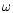 is the dust grain albedo in the far ultraviolet
(typically 0.6 at 150 nm). We note that because CO is destroyed by the
line absorption, its rate of destruction is sensitive to its rotational level
populations. To account for this we have included a temperature-dependence
in the calculation of the rate coefficient.
is the dust grain albedo in the far ultraviolet
(typically 0.6 at 150 nm). We note that because CO is destroyed by the
line absorption, its rate of destruction is sensitive to its rotational level
populations. To account for this we have included a temperature-dependence
in the calculation of the rate coefficient.
For interstellar photoreactions (R2 = PHOTON), the rate is
derived as:
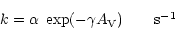 |
(4) |
where 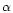 represents the rate in the unshielded interstellar
ultraviolet radiation field,
represents the rate in the unshielded interstellar
ultraviolet radiation field, 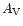 is the extinction at visible wavelengths
caused by interstellar dust, and
is the extinction at visible wavelengths
caused by interstellar dust, and 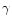 is the parameter used to take into
account the increased extinction of dust at ultraviolet wavelengths.
is the parameter used to take into
account the increased extinction of dust at ultraviolet wavelengths.
3.3 General form of the reaction set
We have re-organised the order of reactions in this release.
The reactions are divided into 14 categories or types, which are grouped
together in the database. Table 8 summarizes these
categories, along with their position in the database. Within each category,
the reactions are listed by increasing total molar mass (total mass of the reactants).
- Photoprocesses: the temperature flags of the photoprocesses, which
are just an artifact from the data revision software, are, of course,
irrelevant.
Also, the unshielded rates shown are valid only for the ISM UV field
(from Draine
1978). These photorates can be rescaled to a stellar radiation field or
calculated from cross sections when available (see Table 7
and Eq. (5));
- Neutral-neutral reactions: the reactions that were already
present in the former UMIST Database have had their temperature dependence
reviewed and temperature range defined when known. When not known, an
arbitrary range of 10 to 300 K has been attributed, since these reactions were
originally defined for these low temperatures;
- Cosmic-Ray reactions: reactions with CRPHOT (cosmic ray photons) and
CRP (cosmic ray protons) have been left
unchanged;
- Sundries: this section gathers all the reactions that cannot be
classified by any of the other types because they are a combination of at
least two different types.
- A major change brought to the database is the inclusion of the
temperature dependence and temperature range for all the reactions. The maximum
temperature range used in the database has been arbitrarily defined from 10 to 41 000 K.
Where explicit information is not available, the rate coefficients have been
attributed a range of 10-300 K. However, if their "
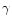 '' Arrhenius coefficient
is too large, the lowest temperature has been defined as
'' Arrhenius coefficient
is too large, the lowest temperature has been defined as
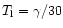 (in K),
and the largest temperature
(in K),
and the largest temperature 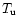 has been arbitrarily taken as 41 000 K (the value of
such rates does not change significantly at higher temperatures), unless the rate's
value becomes unphysical, in which case an appropriate upper temperature has been
determined. Many Ion-Neutral and Ion-Ion reactions remain constant whatever the
temperature, and where this is the case they have been arbitrarily defined from 10
to 41 000 K;
has been arbitrarily taken as 41 000 K (the value of
such rates does not change significantly at higher temperatures), unless the rate's
value becomes unphysical, in which case an appropriate upper temperature has been
determined. Many Ion-Neutral and Ion-Ion reactions remain constant whatever the
temperature, and where this is the case they have been arbitrarily defined from 10
to 41 000 K;
- Two new reaction types have been added, namely Termolecular reactions
and Collider reactions, both of which
become important at high density, typically above
1010 cm-3. Termolecular reactions are catalysed bimolecular
reactions and the catalyst is named "M''. The nature of the third body
is not important in general because it is only used as a de-excitation energy
carrier:
Collider reactions are collision-induced dissociations and the collider
is also named "M'' as its nature does not significantly alter the rate of
the process:
- Because the reactions were chosen to be appropriate not only for the
cold ISM, less
discrimination has been operated so novel reactions have been added to all the
types. The net result is a richer chemistry with multi-product reactions;
- Tables of cross section data appropriate for photo processes have
been gathered and are included in the electronic tables to allow study of
chemistry in a variety of radiation fields. Table 7 gives further information on these cross sections.


Up: The UMIST database for 1999
Copyright The European Southern Observatory (ESO)
![]() ,
,
![]() ,
,
![]() ,
flags
,
flags
![]() ,
,
![]() and
and ![]() are the constants used to
determine the rate coefficients. The series of flags is a string of 16 characters and/or digits that store
respectively:
are the constants used to
determine the rate coefficients. The series of flags is a string of 16 characters and/or digits that store
respectively: